Introduction: t(16;21)(p11;q22)/ FUS::ERG is a rare recurrent abnormality in acute myeloid leukemia (AML) and confers a poor prognosis in AML patients. Previous studies analyzed the role of allogeneic hematopoietic stem cell transplantation (HSCT) in these patients but the results were discordant. AML is characterized by concurrent mutations, which cooperated in the leukemogenesis and refractory/relapse mechanism. However, few studies described the mutational landscape of FUS::ERG AML. Olshanskaya et al. discovered concomitant DNMT3A, ASXL1, RUNX1 and BCOR mutations in the FUS::ERG AML patients but the study was limited to the small case number. Therefore, we aim to assess genomic alterations and clinical outcome of FUS::ERG AML patients with a larger cohort.
Methods: We reviewed medical records of 12328 AML patients diagnosed between December, 2011 and February, 2022 in our hospital. A total of 39 consecutive AML patients with karyotype or reverse transcription-polymerase chain reaction analysis proven t(16;21)(p11;q22)/ FUS::ERG were identified. Whole exome sequencing was performed in 4 patients and targeted sequencing was performed in 20 patients. Patients with follow-up time longer than 30 days were included in the survival analysis. Overall survival (OS) was calculated from the date of diagnosis to the date of death or censoring. Event free survival (EFS) was calculated from the date of diagnosis to the date of refractory, relapse, death or censoring, whichever came first.
Results: M5 was the predominant subtype according to French-American-British classification systems. CD56 (91.9%) and CD123 (81.1%) were found to be expressed in most FUS::ERG AML patients. Six patients had central nervous system involvement diagnosed by flowcytometry (FCM) analysis of cerebrospinal fluid at the time of first lumbar puncture. One patient was diagnosed central nervous system involvement at relapse (Table 1).
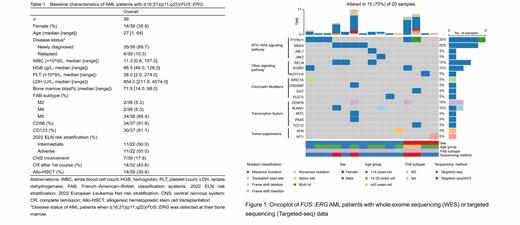
The most prevalent somatic variants of our FUS::ERG AML patients were PTPN11 (25%), NRAS (20%), RELN (15%), CENPB (10%), RUNX1 (10%) and SH2B3 (10%) mutations (Figure 1). Five patients had no somatic mutation detected by targeted sequencing. Notably, RTK-RAS signaling pathway was the most involved pathway after clustering the aberrated genes according to the Gene Ontology terms, which included PTPN11, NRAS, ABL1 and JAK2 aberrations. All but one PTPN11 mutations affected the amino terminal (N)-SH2 domain. NRAS mutation were found mutated at amino acid G12 and Q61.
The median EFS of the patients was 9.3 months (95% CI: 9.2-NA) and OS was 18.2 months (95%CI: 13.1-NA). Due to the poor outcome of this subtype of AML, allogeneic HSCT was highly recommended. A total of 14 patients underwent allogeneic HSCT. Notably, seven of ten patients were positive for minimal residual disease assessed by FCM at the time of transplantation. We did not observe the statistically significant effect of HSCT on improving the EFS (p = 0.24) or OS (p=0.26) of our patients.
Conclusions: PTPN11 and NRAS were the most frequent mutations and RTK-RAS signaling pathway was the most involved pathway in rare AML patients with t(16;21)(p11;q22)/ FUS::ERG. The addition of signaling pathway inhibitors followed by HSCT might be an effective strategy to overcome the dismal outcome of this subtype. Larger cohort studies are warranted to investigate the molecular characteristics further as well as evaluate the clinical activity of the SHP2 (PTPN11) inhibitors in FUS::ERG AML patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal